Abstract
Introduction:
Over the past ten years, the treatment of Acute Myeloid Leukemia (AML) has substantially changed. New therapies emerged, and with the advance in understanding the genetic and molecular basis of AML, we incorporated new biomarkers into the standard evaluation of patients. The 2022 ELN recommends immunophenotyping, cytogenetics, and molecular studies as the basic initial testing of any new patient. Those are necessary to establish risk stratification and diagnosis. In our retrospective study, we looked into whether we would have decided to change therapies or had more options presented to a patient in case we had molecular and cytogenetics studies upfront and before starting treatment.
Methods:
We collected data from patients with AML treated at our university hospital from June 2020 to June 2022. The specific information we captured was: blood counts on admission, ELN risk stratification, Charleston score, type of treatment, time to cytogenetics results, time to Next Generation Sequence results, the best response to therapy, MRD status, and consolidation with allogeneic stem cell transplant.
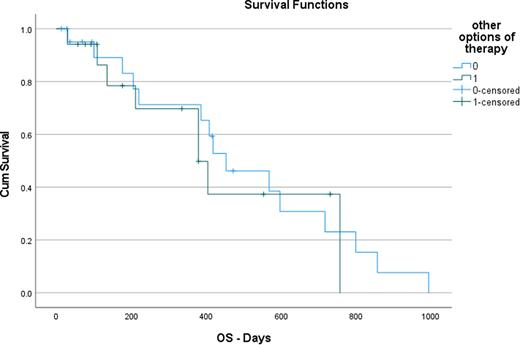
After we collected all information, each patient had a reevaluation. If a patient had any of the following molecular/cytogenetics changes (FLT3, cytogenetics changes associated with myelodysplasia, p53 mutation, complex karyotype, BCR-ABL1, RUNX1-RUNX1T1, CBFB-MYH11, Variant-type PML/RARA), he was carefully reevaluated and included in the "other options of therapy" subgroup. We included 17 patients in the "other options of therapy" subgroup and twenty-two in the "standard" group.Results: We included 39 individuals. The mean age of diagnosis was 62.18 (27-83). Three patients were stratified as good ELN risk, 17 as adverse risk, and 19 as intermediate risk. The mean Charlson Comorbidity Index (CCI) was 5.51 (2-10); 17 patients received high intense chemotherapy and 22 low intense chemotherapy. The mean wait to start therapy was of 7 days. The mean waiting time for NGS results was 12.71 and 7.30 days for cytogenetics. Seventeen patients were classified in the "other options of therapy" group. And twenty-two on the "standard" group. In the standard group, the mean overall survival was 496.5 days, and on "other options of therapy" it was 447.7. There was no statistically significant difference in survival between the two groups (p=0.602). (figure 1)The main reason for other therapy options was the presence of myelodysplastic genes (3), tp53 (3), RUNX1-RUNX1T1(2), CBFB-MYH11(1), cytogenetics suggesting myelodysplastic changes (6), Variant-type PML/RARA (1) and FLT3 (3).
Conclusions:
In this study, we retroactively evaluated if having more initial therapy options would have impacted the overall survival of newly diagnosed AML patients. Our survival analysis showed no significant statistical difference between the two groups. The median time to results of NGS was 12.71, and for cytogenetics, 7.3 days. In our cohort, the median time for therapy from diagnosis was 7.00 days. The reasons to change therapies were: midostaurin on FLT3 patients, Gemtuzumab ozogamicin on good risk patients, CPX-351, or alternative induction strategies in patients with p53 or unfavorable cytogenetics, and alternative induction on patients with Variant-type PML/RARA. This study has limitations due to its retrospective nature, small population, and short follow-up period.
Disclosures
No relevant conflicts of interest to declare.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal